We don't need CATL to tell us how they achieved 500 Wh/kg battery, we know [Part 1]
Is there an optimal chemistry that the industry is converging towards?
In April 2023, the Chinese public corporation CATL announced a breakthrough in battery technology: a lithium-ion battery with a specific energy of 500 Wh/kg. This is almost twice as much as the best commercial batteries available today. Such a battery could revolutionize electric vehicles, especially electric aviation, by enabling longer ranges at lower costs.
But what is the secret behind this potential breakthrough? How does this newly-announced “condensed matter” battery compare to Amprius’s recent innovation that was also claimed to have 500 Wh/kg of specific energy?
Unfortunately, CATL remained tight-lipped about the chemistry or structure of this new battery. This is not ideal for us as investors as we’d like to understand what direction such players like CATL have taken to be able to anticipate what’s coming. The good news is that we can deduce a lot based on the fundamental chemistry of Li-ion batteries. In the following post (the first one in a series), we put together a comparison of the theoretical limits of various battery chemistries. That also allows us to speculate whether all battery manufacturers will eventually converge on more or less the same combination of cathode, anode, and electrolyte to get maximum specific energy.
Simplistically as evident by the name of the battery type it all boils down to how much ions of Li might be stored and moved between anode and cathode. The table below shows the capacities of various cathodes Ccat (horizontal axis) and anodes Can (vertical axis) based on their chemical structure and masses of all atoms in the electrode per one atom of lithium. It assumes complete lithiation/delithiation during the charging and discharging. As capacities represent charge divided by mass, they are measured in mAh/g. The masses are normalized to the same charge unit for both cathode and anode as the number of lithium atoms transferred from the cathode is the same as the number of atoms transferred to the anode: ions do not accumulate in the electrolyte.
In other words, we use Faraday’s laws of electrolysis, which in this case implies that the mass of lithium deposited at an electrode is directly proportional to the charge transferred. In the table itself, we calculate the capacities C of combinations of various cathodes and anodes. To do so, we sum the reciprocals of capacities of the cathode and anode. As reciprocals represent mass divided by charge (measured in g/mAh), we are effectively summing up masses of cell components per unit of charge transferred by Li ions. This leads to the formula:
(here by including the last term, we effectively subtract pure lithium mass from the total cell mass to avoid double counting, as Li is already included in both cathode and anode capacities).
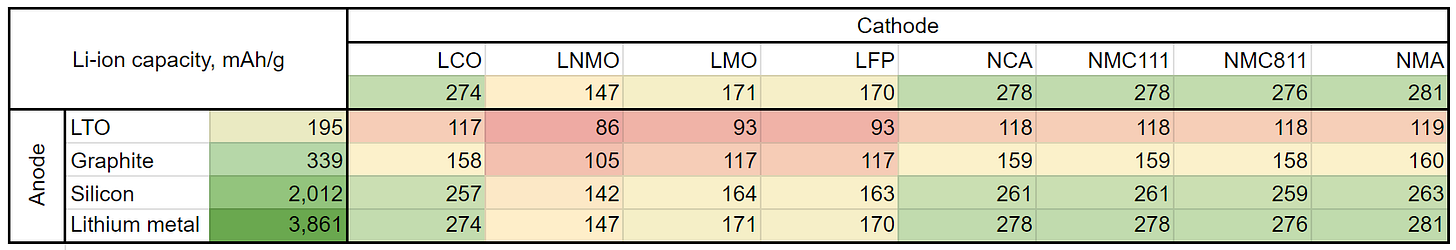
It's worth noting that the table doesn't consider that some compounds may be unstable when combined. Moreover, when comparing different combinations, it's essential to take into account, the cell voltage, the weight of the separator, current collectors, and electrolyte which is not done at this approximation. So, this table is an upper limit of capacities for each combination, which provides us with a useful illustration of what anode/cathode compounds are more promising for higher energy capacities.
In order to calculate the specific energy in Wh/kg, these capacities must be multiplied by the cell voltage, which typically is in the range of 3-4 V. Take, for example, the NMA811 + lithium metal, operating at a voltage of 3.80 V, thus enabling a theoretical specific energy of a staggering 1,049 Wh/kg. When components like the separator, current collectors, and an electrolyte are factored into the equation, this figure drops to 500 Wh/kg tops. Interestingly, this is the same ambitious target pursued by companies such as Amprius, CATL, and many others in the battery world.
A quick glance at each cathode/anode pair in the table shows that the level of 500 Wh/kg is attainable when employing Ni cathodes such as NCA, NMC, and NMA in combination with either a silicon or lithium metal anode (bottom right corner of the table, colored green). The other combinations either yield lower specific energy or necessitate the use of costly materials. LCO cathode serves as an excellent example of this. In theory, it facilitates high capacity but comes at the price of expensive cobalt.
It seems that the 500 Wh/kg mark stands as a practical limit for Li-ion technology, a challenging but achievable goal in the coming years. Breaching this barrier will demand the exploration of less established chemistries, such as lithium-sulfur or lithium-air. These areas are still full of many unsolved problems even at the laboratory prototype stage. Thus we believe the industry giants like CATL are focused now on optimizing more proven chemistries represented at the bottom right of our table.
Worth noting here, that such capacities will only be required for energy-intensive applications (such as Electric Aircraft, EA), so the majority of Li-ion batteries produced in the next five years will be based on much cheaper variations. Like, for example, cells comprising a lithium iron phosphate (LFP) cathode that despite having a lower capacity, boast a longer cycle life and are much cheaper to produce. This makes them suitable for electric vehicles and perfect for grid storage, alongside their sodium-based counterparts.
All of the above suggests that for applications where maximum specific energy is critical, like EA, reliance will likely be on Ni cathode and silicon or lithium metal anode. Stay tuned as we are going to explore this in detail in further posts.